BioLASCO Taiwan Co., Ltd.
3F., No. 267, Chongyang Rd., Nangang Dist., Taipei City, , Taiwan (R.O.C.)
Tel:(02) 2651-8669
Fax:(02) 2651 – 8969

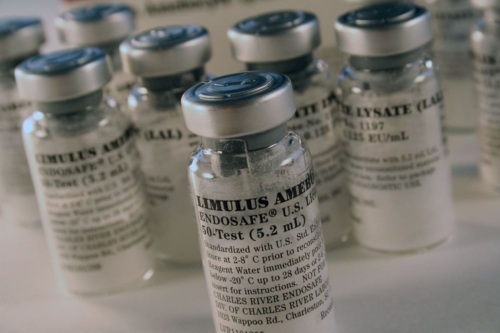
Limulus amebocyte lysate (LAL), an aqueous extract derived from Limulus amebocytes, which is intended for quantitative detection of endotoxins by the Gel-clot methods. Improvements in LAL reagents, the advent of standard methods and automated systems, and a better understanding of LAL reactivity make the LAL reagent readily adaptable to testing a variety of biologics, parenteral products and medical devices. Currently, endotoxin detection methods based on lysate reagents include the Gel-Clot, Turbidimetric assay, and Chromogenic assay.
CRL Endosafe® endotoxin testing reagent has been approved by the FDA. Our gel clot endotoxin test lysate features a firm gel over a wide range of sensitivities, designed with advanced formulations to provide better interference resistance and higher accuracy for routine LAL testing.
The basis of the test is that endotoxin produces an opacity and gelation in LAL that is easily recognized. With the aid of a microprocessor and microplate reader, a kinetic turbidimetric assay may be done where the early onset of turbidity, which precedes gelation, can be detected and precisely measured. The time for onset of turbidity is inversely related to the amount of endotoxin in the sample, so endotoxin levels in unknown samples are determined by comparison to a standard curve. With kinetic measurements, lambda (λ) is the lowest point on the standard curve.
KTA can be used for both kinetic and gel clot analysis, which reduces the issues associated with switching between the two methods.
KTA2 offers faster reaction times without the need for a pre-incubation period. It also provides a wide standard curve (to 0.005 EU/mL).
| Kinetic Turbidimetric Reagents | Sensitivity (EU/mL) | Code | Format |
| KTA2 | * | R19000 |
Box of 100 |
| KTA | * | R15015 | |
| * | R15003 | ||
| * | R15006 |
* The detection concentration range is 50-0.005 EU/mL, but with the use of appropriate instruments and consumables, a wide standard curve can be achieved (to 0.001 EU/mL).

The E. coli CSE is a validated positive control, standardized and quantified using the U.S. Reference Standard Endotoxin. For detailed verification, please refer to the Certificate of Analysis (CoA).
| Control Standard Endotoxin | Code | Format |
| CSE-10 ng per vial | E120 | Pack of 6 |
| CSE-500 ng per vial | E110 | Pack of 6 |
| Reference standard endotoxin (RSE) | E150 | ─ |

To prevent contamination, all accessories and consumables must be endotoxin-free. Reagents and test samples should be prepared using endotoxin-free water for bacterial endotoxin testing.
| LAL Reagent Water | Code | Format |
| 50 mL bottle | W120 | Case of 12 |
| 100 mL bottle | W110 | Case of 12 |
| 500 mL bottle | W150 | Pack of 6 |
* Endotoxin Test Result : < 0.001 EU/mL.
3F., No. 267, Chongyang Rd., Nangang Dist., Taipei City, , Taiwan (R.O.C.)
Tel:(02) 2651-8669
Fax:(02) 2651 – 8969