BioLASCO Taiwan Co., Ltd.
3F., No. 267, Chongyang Rd., Nangang Dist., Taipei City, , Taiwan (R.O.C.)
Tel:(02) 2651-8669
Fax:(02) 2651 – 8969
Charles River is dedicated to the sustainable development of horseshoe crabs, continuously seeking methods to reduce or replace technologies while ensuring safe for patients around the globe. This commitment has led to the creation of a globally unique Portable Endotoxin Testing System and the development of Endosafe® Trillium™ recombinant protein reagents through genetic engineering, thereby reducing reliance on horseshoe crabs.
For natural environmental endotoxins, our proprietary matrix ensures accurate recovery of endotoxins and demonstrate superior performance in comparability and stability. This not only delivers precise and reliable endotoxin detection results but also reinforces sustainable development efforts.
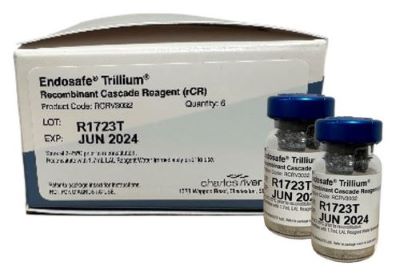
A robust, animal-free recombinant cascade reagent (rCR), Endosafe® Trillium™ is the latest addition to our trusted and industry-leading Endosafe portfolio, and was developed with superior science, and designed for sustainable progress in the lab.
The formulation and composition of Trillium includes the three critical biological proteins (recombinant Factor C, recombinant Factor B, and recombinant proclotting enzyme) and a specific concentration of key components. As an optimized kinetic chromogenic reagent curated to simulate the natural LAL reaction, it offers high specificity and eliminates the risk of 1,3-β-D-glucans interference.
| Endosafe® Trillium™ | Sensitivity (EU/mL) | Code | Format |
| 32-test vial (1.7 mL) | 0.005 | RCRV3032PK | Pack of 6 |

The E. coli CSE is a validated positive control, standardized and quantified using the U.S. Reference Standard Endotoxin. For detailed verification, please refer to the Certificate of Analysis (CoA).
| Control Standard Endotoxin | Code | Format |
| CSE-10 ng per vial | E120 | Pack of 6 |
| CSE-500 ng per vial | E110 | Pack of 6 |
| Reference standard endotoxin (RSE) | E150 | ─ |

To prevent contamination, all accessories and consumables must be endotoxin-free. Reagents and test samples should be prepared using endotoxin-free water for bacterial endotoxin testing.
| LAL Reagent Water | Code | Format |
| 50 mL bottle | W120 | Case of 12 |
| 100 mL bottle | W110 | Case of 12 |
| 500 mL bottle | W150 | Pack of 6 |
* Endotoxin Test Result : < 0.001 EU/mL.
3F., No. 267, Chongyang Rd., Nangang Dist., Taipei City, , Taiwan (R.O.C.)
Tel:(02) 2651-8669
Fax:(02) 2651 – 8969